Central Regulation of Metabolism in the Pers Group
The Pers Group conducts cutting-edge research on the molecular mechanisms underlying obesity and type 2 diabetes susceptibility, particularly focusing on brain-centric molecular pathways influenced by polygenic risk. We integrate advanced machine learning, cutting-edge variant-to-function techniques from human genetics, and CRISPR-based genome engineering to uncover novel insights. To validate our hypotheses, we employ physiologically relevant transgenic rodent models, bridging the gap between molecular discoveries and real-world therapeutic applications.

The overarching aim of the Pers Group is to identify molecular pathways in the brain mediating genetic susceptibility to metabolic disease.
Obesity and type 2 diabetes are a heritable multifactorial disease with incompletely understood etiologies. The Pers group investigates the role of the brain in the control of metabolic fuel homeostasis with a focus on how polygenetic liability in energy and blood glucose-regulatory systems contribute to the pathogenesis of obesity and diabetes. The group leverages computational techniques, human genetics and single-cell sequencing data, pharmacological and physiological studies to explore how rodent and human brain cell populations are involved in the response to an array of humoral signals, including FGF1, GLP-1, leptin, insulin, and nutrients such as glucose.
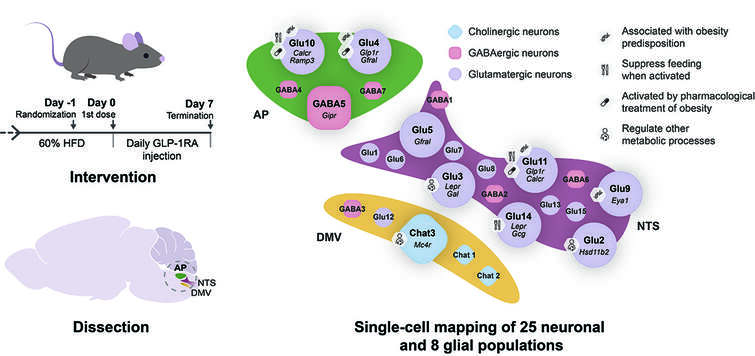
“A genetic map of the mouse dorsal vagal complex and its role in obesity”
Published in Nature Metabolism in 2021, this article presents a transcriptional and chromatin-accessibility single-cell atlas of the mouse dorsal vagal complex and uses body mass index genome-wide association data and Designer Receptors Exclusively Activated by Designer Drugs (DREADD)-based chemogenetic tools to show that some of them control feeding.
“Transcriptomic analysis links diverse hypothalamic cell types to fibroblast growth factor 1-induced sustained diabetes remission”
Published in Nature Communications in 2020, this article uses single-cell RNA sequencing and histological techniques to show that sustained diabetes remission induced by intracerebroventricular fibroblast growth factor 1 is dependent on intact melanocortin receptor 4 signaling.
"Genetic mapping of etiologic brain cell types for obesity"
Published in eLife in 2020 this study integrates published single-cell RNA-sequencing data from 727 peripheral and nervous system cell types spanning 17 mouse organs with body mass index genome-wide association study data to pinpoint brain cell populations likely mediating polygenetic for obesity.
Funding - Principal Investigator on external grants
- 2019 – 2022 PI: Danish Research Council for Independent Research Sapere Aude Starting Grant: ‘Hypothalamic plasticity in obesity’ (EUR 781,350)
- 2016 – 2018 PI: Novo Nordisk Foundation research grant for basic and clinical research in endocrinology and experimental physiology: ‘Identification and characterization of GLP-1 responding hypothalamic cell types’ (EUR 187,500)
- 2015 – 2020 PI: Lundbeck Foundation Fellowship: ‘Understanding weight gain by uncovering shared biology between schizophrenia and obesity’ (EUR 1,339,700)
- 2013 – 2015 PI: Benzon Foundation Postdoc Fellowship: ‘Systems genetics in obesity: Integration of association data from 350,000 individuals with cell type-specific hypothalamic expression data’ (EUR 93,750)
- 2011 – 2013 PI: Danish Research Council for Independent Research | Medical Sciences postdoc fellowship: ‘Systems genetics in obesity: Common and rare variant data analysis for identification of risk variants and susceptibility pathways’ (EUR 364,067).
Honors and awards
- 2019 Danish Diabetes Academy Young Investigator Award.
- 2017 Jakobiner Society honorary medal for young researchers below 40 years.
- 2016 – present Member of the Young Academy of the Royal Danish Academy of Sciences and Letters.
- 2011 The Danish Council for Independent Research Sapere Aude award for 'the best young researchers at Postdoc level'.
Current management Activities
- Part of the CBMR center leadership team.
- Steering group member of the University of Copenhagen Center for Health Data Science.
- Advisory board of the Single-Cell Omics Platform at CBMR.
International Collaborations:
Tune H. Pers is principal investigator at the Novo Nordisk Foundation Center for Genomic Mechanisms in Disease, and he is a part of the Genetic Investigation of ANthropometric Traits (GIANT) and the International Common Disease Alliance (ICDA) consortia. In addition, he closely collaborates with principal investigators at the University of Washington (Seattle, USA), the University of Michigan (Ann Arbor, USA) and Oxford University (Oxford, UK).
Group Leader
Tune H. Pers
Associate Professor
Phone: +45 3533 5755
tune.pers@sund.ku.dk
Twitter:
Tune H. Pers (@tunepers)

Staff list
| Name | Title | Phone | |
|---|---|---|---|
| Andersen, Charlotte Høy | Academic Research Staff | ||
| Belmont-Rausch, Dylan Matthew | Postdoc | +4535326151 | |
| Borgmann, Diba | Postdoc | ||
| Brown, Jenny Marie | Postdoc | +4535334806 | |
| Christensen, Oliver Pugerup | Master Student | ||
| Coester, Bernd | Postdoc | +4535328008 | |
| Egerod, Kristoffer Lihme | Research Consultant | +4535337057 | |
| Fan, Yong | Assistant Professor | +4593509187 | |
| Hassan, Shad | Academic Research Officer | +4535323938 | |
| Laurentiussen, Claes Ottzen | Master Student | ||
| Lilja-Fischer, Helle Kinggaard | Laboratory Technician | +4526719296 | |
| Lyu, Liwei | Visiting PhD Student | +4593509186 | |
| Mottelson, Noah Wulff | PhD Fellow | ||
| Pers, Tune H | Associate Professor - Promotion Programme | +4535335755 | |
| Stannius, Tobias Overlund | Guest Researcher | ||
| Thomas, Cecilia Engel | Postdoc | ||
| van Hilten, Arno | Research Assistant | +4535321319 |