Control of Metabolic Homeostasis in the Gerhart-Hines Group
The Gerhart-Hines Group interrogates peripheral and central mechanisms of homeostatic control and seeks to leverage their discoveries to develop novel pharmacotherapies for the treatment of obesity and type 2 diabetes.

Bottom row (left to right): Fabian Finger, Katrine Wonsbek, Cecilie Kynding Kristensen, Camilla Trang Vo, María Gestal Mato
Middle row (left to right): Hannes Embring Elofsson, Katja Munch Lorentsen, Astrid Linde Basse, Frederike Sass, Zach Gerhart-Hines
Top row (left to right): Ayat Khaled Fayez Albadri, Rebecca Lynn McIntyre, Camilla Honoré Grauslund, Sofie Lund Polat, Lucile Dollet, David Tandio
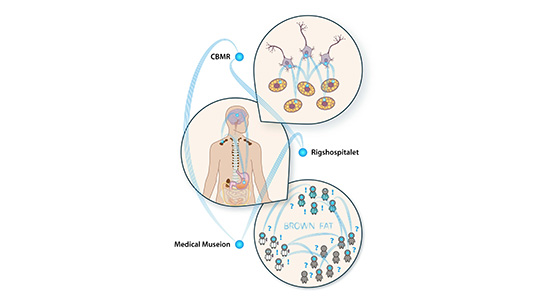
We are broadly interested in how neuronal, hormonal, nutrient, and bioenergetic signalling pathways collectively orchestrate systemic energy homeostasis and defend metabolic health. We specifically focus on adipose tissue and, more recently, the dorsal vagal complex (DVC) as paradigms of peripheral and central control. By exploring peripheral and central homeostatic pathways through a combinatorial approach that brings together gene, protein, metabolite, and lipid profiling with cutting-edge in vivo physiological phenotyping and pharmacological engineering, we believe we are poised to make transformative breakthroughs in the fundamental understanding of metabolic adaptation and to develop these findings into innovative strategies for counteracting cardiometabolic disease.
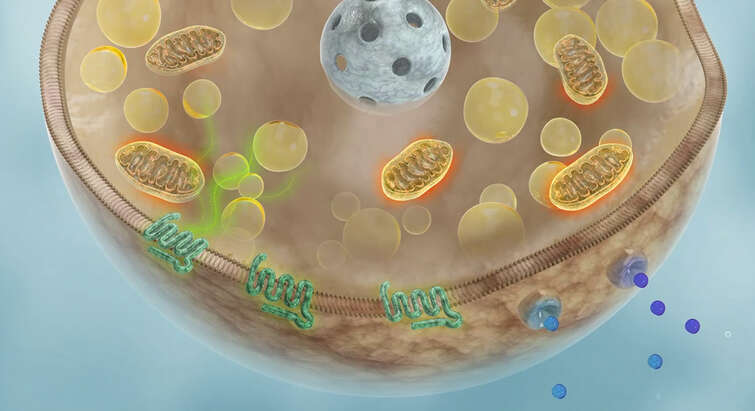
In adipose tissue, we seek to understand the signals and cues from the environment, diet, circadian clock, and other organs that shape function and tissue plasticity. The overarching goal of this arm of our research is to uncover how these diverse 'inputs' converge on adipocytes to uniquely shape adipose tissue biology and coordinate organismal energy metabolism. Specifically, we focus on identifying cell surface receptors, intracellular enzymes, and transporters that represent key regulatory nodes in influencing adipose tissue catabolism. We also have a long-standing interest in mitochondrial biology, particularly how changes in bioenergetic capacity are communicated to the nucleus and other organelles in the adipocyte and even between tissues.
Our work in the DVC was initially inspired by our discovery of a new appetite-suppressing receptor pathway that integrates signals from the periphery to mediate non-aversive control of food intake through novel hindbrain circuits. Interestingly, in parallel to central appetite regulation, activation of this receptor in the periphery increases energy expenditure and enhances insulin sensitivity.
Underscoring the potential of our approach, the first company spun out from my group, Embark Biotech, which was centered around this novel peripheral and central receptor pathway, was acquired in August 2023 by Novo Nordisk. My co-founders and I have now formed, Embark Laboratories, to continue the collaboration with Novo Nordisk to pioneer the development of next generation therapeutics for improving the lives of people living with obesity and type 2 diabetes.
"Lipolysis drives expression of the constitutively active receptor GPR3 to induce adipose thermogenesis"
Published in Cell in 2021. G protein-coupled receptors (GPCR) are classically regulated by the binding of a soluble ligand. In this study, we found that the constitutively active receptor, GPR3, was instead regulated at the transcriptional level by a noncanonical signal from lipolysis in brown adipocytes. Upon reaching the cell surface, GPR3 begins signalling through Gs proteins to increase cAMP levels without the apparent need of a ligand. Thus, increasing Gpr3 expression is fully sufficient to drive the downstream hallmarks of thermogenesis in mouse and human adipocytes. These findings open up new avenues through which we hope to harness and engineer adipose metabolism for therapeutic purposes.
“NAMPT-mediated NAD+ biosynthesis is indispensable for adipose tissue plasticity and development of obesity”
Published in Molecular Metabolism in 2018. In this study, we show for the first time that the NAD+ biosynthetic enzyme, NAMPT, is an essential regulator of adipose expansion. Genetic depletion of NAMPT in adipose tissue completely prevents mice from gaining weight and becoming obese even on a diet rich in fat.
"Cardiolipin Synthesis in Brown and Beige Fat Mitochondria Is Essential for Systemic Energy Homeostasis"
Published in Cell Metabolism in 2018. In this study, we found that synthesis of the mitochondrial phospholipid cardiolipin is indispensable for stimulating and sustaining thermogenic fat function. Additionally, we unexpectedly discovered a novel role for cardiolipin in mediating communication from the mitochondria to the nucleus as an indicator of respiratory capacity. Finally, this was the first study to demonstrate that acute mitochondrial dysfunction in brown fat caused whole-body insulin resistance.
"A novel endocrine axis that potently induces energy expenditure and weight loss"
Our investigation of adipose energy-expending pathways led to the discovery of a new regulator of organismal homeostasis. Targeting this pathway is capable of counteracting obesity and related disorders in rodent models of metabolic disease. In 2018, we spun out Embark Biotech from the University of Copenhagen to fully explore the innovative potential of this candidate as an obesity drug.
Wolfrum C, Gerhart-Hines Z. Fueling the fire of adipose thermogenesis. Science. 2022 Mar 18;375(6586):1229-1231. doi: 10.1126/science.abl7108. Epub 2022 Mar 17. PMID: 35298244.
Group Leader
Zach Gerhart-Hines
Associate Professor
Phone: +45 6067 0682
zpg@sund.ku.dk
Twitter:
Zach Gerhart-Hines (@Z_GerhartHines)