A cell atlas of the brainstem identifies cells that regulate obesity
Scientists at the University of Copenhagen have characterized lower brainstem cell populations through which new and promising anti-obesity drugs likely act to reduce appetite. The ‘unbiased’ approach identified the cell populations responsible for regulating appetite using bioinformatics and single-cell sequencing tools. The scientists believe the approach will unlock more secrets about the neurological control of appetite and energy balance.
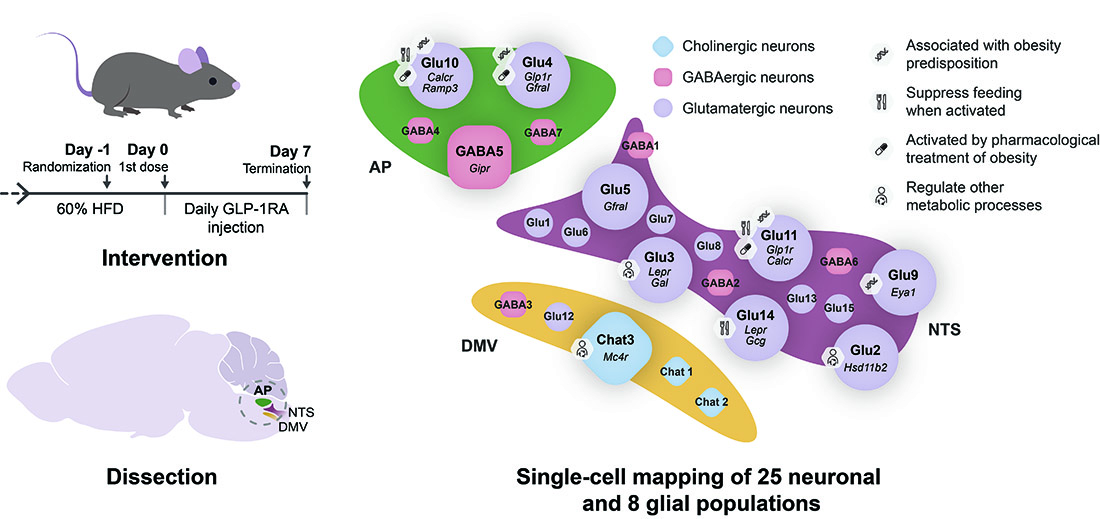
While the brainstem is known to play a key role in obesity, the molecular identities of the specific brain cells that exert an influence on body weight regulation with a few exceptions yet have to be characterized. Scientists at the University of Copenhagen have now successfully identified some of these cells by mapping the gene expression of cells in the mouse lower brainstem, and cross-referencing the data with human genes that are known to predispose people to obesity. They found that some of the cell populations that they identified in the lower brainstem are already being targeted by new and promising anti-obesity medications.
Rising rates of obesity globally has accelerated work to find effective medications. Research increasingly suggests that the brain plays an important role in regulating our appetite and body weight. Genetic research, in particular, has shown that genetic risk variants associated with obesity are near genes that are predominantly expressed in the brain.
“We know that the brain plays a big role in energy regulation, however, apart from some prominent examples that are predominantly the hypothalamus, we lack insights into which cells are responsible,” says Associate Professor Tune H Pers from the Novo Nordisk Foundation Center for Basic Metabolic Research (CBMR) at the University of Copenhagen, who published the research in Nature Metabolism.
Profiling mouse brain cells helped identify brain cells that play a role in obesity
He and PhD student Mette Q Ludwig set out to better understand which cells in the lower brainstem control energy balance. They first treated obese mice with some of the most effective anti-obesity drugs, which mimic the hormone GLP-1, called GLP-1 receptor agonists. They examined how the drugs changed gene expression in different areas of the brain and identified a specific area in the brainstem called the area postrema that was most affected.
They then zoomed in on the area postrema and its neighboring areas, which are together called the dorsal vagal complex. Using detailed single-cell sequencing tools, they mapped 25 distinct populations of neurons within that area. Two populations that contained receptors for GLP-1 – which were activated by the GLP-1 receptor agonists – expressed genes known to reduce appetite. Another population expressed receptors for the hormone amylin, which is already being explored by pharmaceutical companies as a possible anti-obesity drug.
They then cross-referenced this atlas against known genetic variants that are associated with obesity in humans. They found that cell populations that expressed receptors for GLP-1 and amylin, also expressed genes that are linked to obesity.
“We were surprised how well our approach based on single-cell profiling of mouse brains actually allowed us to identify likely relevant cell populations for human obesity,” says Mette Q Ludwig.
The promise of unbiased and data-driven science
To test the idea, they collaborated with Professor Martin G Myers from the University of Michigan to carry out experiments in genetically modified rats where they specifically activated neurons that have amylin receptors. The food intake of these rats was significantly reduced, confirming their hypothesis that these neurons suppress appetite.
This research provides new insight on how anti-obesity drugs work by identifying the cells they activate in the brain. The findings are notable, as the scientists used advanced data science methods to unbiasedly look at many areas and cell populations in the brain, rather than seeking to specifically validate predefined hypotheses.
“In this work, we primarily identified brain cell populations that are already being targeted by promising new anti-obesity medications. I am, however, convinced that we can use our data-driven approach over the next few years to identify additional brain cell populations that potentially hold key roles in body weight regulation,” says Tune H Pers.
Read the full article in Nature Metabolism here: 'A genetic map of the mouse dorsal vagal complex and its role in obesity'
Contact
Associate Professor Tune H Pers
Phone +45 3533 5755
tune.pers@sund.ku.dk
