Research Program 3
Physiological Control of Metabolic Homeostasis
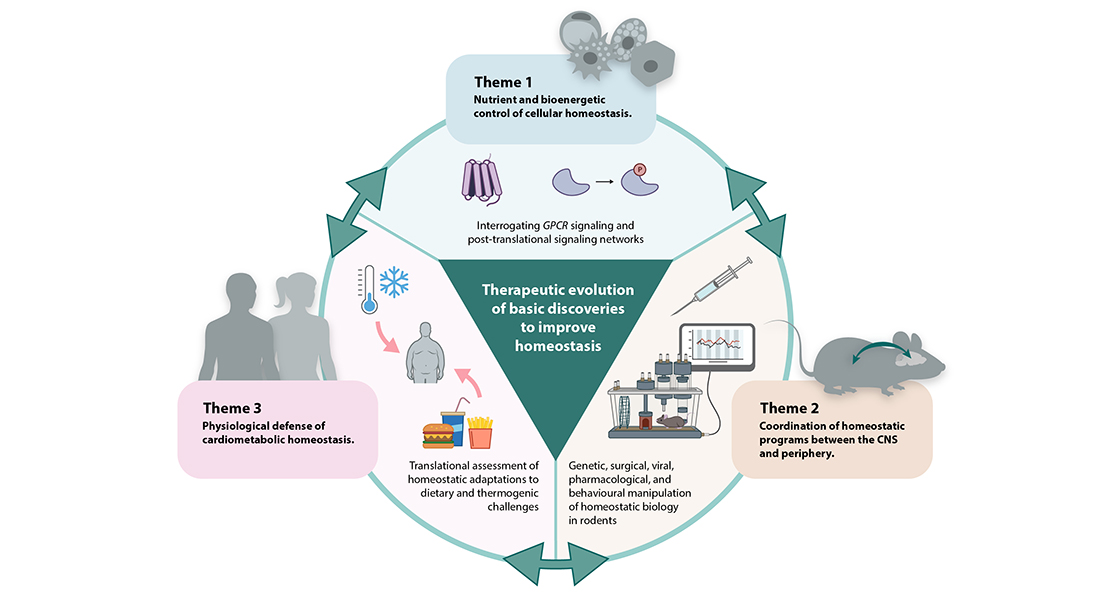
The goal of this Research Program is to identify the protective signaling networks that underlie homeostatic control of metabolism at the molecular, cellular and tissue levels and determine how these mechanisms are integrated to coordinate whole-body energy balance.
Scope
Building on fundamental discoveries of new or previously unappreciated physiological networks and factors, Program 3 will pursue the development of cutting-edge, innovative pharmacotherapies for counteracting cardiometabolic indications.
Energy homeostasis is a core element of cardiometabolic health, and breakdown of any of these evolutionarily fine-tuned defenses leads to a litany of disease states across organ systems. A set of key challenges is to determine how cells in different tissues sense intrinsic homeostatic perturbations (e.g., mitochondrial dysfunction), and then signal-cell autonomously, to counteract or adapt to such perturbations, as well as how cells convey the homeostatic disruption to surrounding cell types within the organ (i.e., paracrine) and throughout the body (i.e., endocrine). In addition to investigating how these cellular homeostatic functions are mediated within peripheral tissues, including adipose tissue, skeletal muscle and liver, Program 3 will also interrogate the bidirectional coordination between tissue-specific metabolic programs and the CNS.
As highlighted below, several research foci within the Program have integrated a CNS focus on homeostatic control. These efforts will be further strengthened through collaboration with Professor Ole Kiehn at the Department of Neuroscience as well as established international Research Alliances (Professors Martin G. Myers Jr. at University of Michigan and Michael Schwartz at University of Washington). Finally, Program 3 will seek to investigate homeostatic paradigms in humans within different physiological contexts, including temperature and dietary challenges.
The scope of Program 3 does not extend to homeostatic disruption or pathological energy imbalances leading to beta-cell failure/type 1 diabetes.
Nearly all cells in the body are equipped with measures to sense homeostatic disruptions such as nutrient availability or mitochondrial oxidative stress. Some of these adaptive programs are conserved across cell types and some are specialized to the unique metabolic features of a particular organ system. Cells experiencing homeostatic imbalance are often capable of actively conveying this metabolic emergency to surrounding cell types and other tissues to help counteract the dysfunction and preserve organismal fitness. Breakdown in the sensing, signaling, or relaying cues in tissues, such as skeletal muscle, liver or adipose tissue, can result in metabolic dysfunction. By focusing on cell surface receptors and kinase signaling cascades and secreted mediators of homeostatic cues, Program 3 seeks to uncover key cell-specific nodes of metabolic control that would serve to expand the understanding of protective biological mechanisms and reveal novel targetable strategies to improve cardiometabolic health.
Mammalian body weight is regulated by biological mechanisms that match energy intake to energy expenditure. Sensing of circulating “metabolic messengers” by the CNS is a key feature of this control system and the subsequent behavioral and metabolic responses initiated by the brain allow animals and humans to maintain fat mass within a range that supports survival. Energy-need based hormonal and neural signals are sensed by the hypothalamus and the brainstem. Homeostasis is maintained through complex interconnected neural circuitries implicating motivation and reward as well as neuropsychological processes such as cognition. The pathological insults that lead to subtle, but chronic energy imbalances in this fine-tuned control system, are incompletely understood.
Across Research Programs, scientists at CBMR, in collaboration with leading neurophysiologists and neuroendocrinologists (Professors Ole Kiehn, Martin Myers and Michael Schwartz), have started to systematically decode neural components of energy homeostasis and decipher the integrative regulation of such pathways at the whole-body level. The interrogation of the central regulation of energy homeostasis ties into the strong focus on therapeutic innovation of Program 3, towards identifying and translating novel druggable pathways for treatment of disorders.
Program 3 will extend the causal mechanistic studies of homeostatic control in cells and murine tissues to human physiology. Researchers in the Program will address outstanding knowledge gaps regarding adaptation to paradigms such as temperature and excess caloric challenges through tissue-specific gene, lipid, metabolite and secretomic profiles in adipose tissue and skeletal muscle, as well as the physiological assessment of body composition, respiration, appetite and glucose tolerance. These studies will be supported by retro-translational investigation in rodents to characterize mechanisms and signaling pathways mediating homeostatic responses.
By investigating the physiological responses in humans to different homeostatic perturbations from the cell to whole-body level, Program 3 will be able to uncover and prioritize the most translationally relevant regulatory networks driving energy balance.


